Frustrated with CE Cetificate Documentation?
Clinical Evaluation Report (CER) as per MEDDEV 2.7.1 Rev. 4
MED DEV 2.7.1 Rev. 4 is in full force. If you haven't started the process of updating your existing Clinical Evaluation Report (CERs), your CE Certification will be in trouble and chances of getting Notified Body suspension letter.
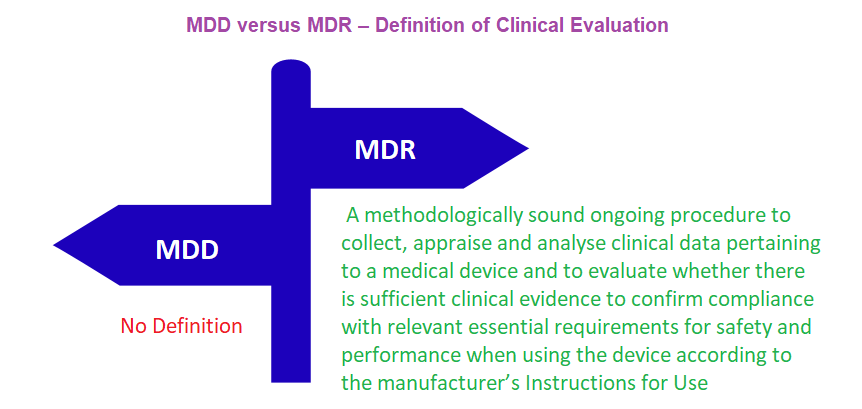
Clinical evaluation Report conclusions made to demonstrate safety and performance of the device from the evaluation, with respect to the intended use of the device
The justification derived to state whether
- Clinical evidence demonstrates conformity with relevant Essential Requirements
- Performance and safety of the device as claimed have been established
- Risks associated with the use of the device are acceptable when weighed against the benefits to the patient
Clinical Evaluation Report contents
Clinical Evaluation as per MEDDEV 2.7.1 Rev. 4
LIBERTY MANAGEMENT GROUP LTD.
Chicago
75 Executive Drive, Suite 114
Aurora, IL - 60504
Phone : (630) 270-2921
Fax : (815) 986-2632
E-mail : info@libertymanagement.us
New York
125 Woodhill Lane
Manhasset, NY 11030
Phone : (516) 244-2376
E-mail : newyork@libertymanagement.us